Recruitment & Enrollment – PCaP Baseline
Enrollment
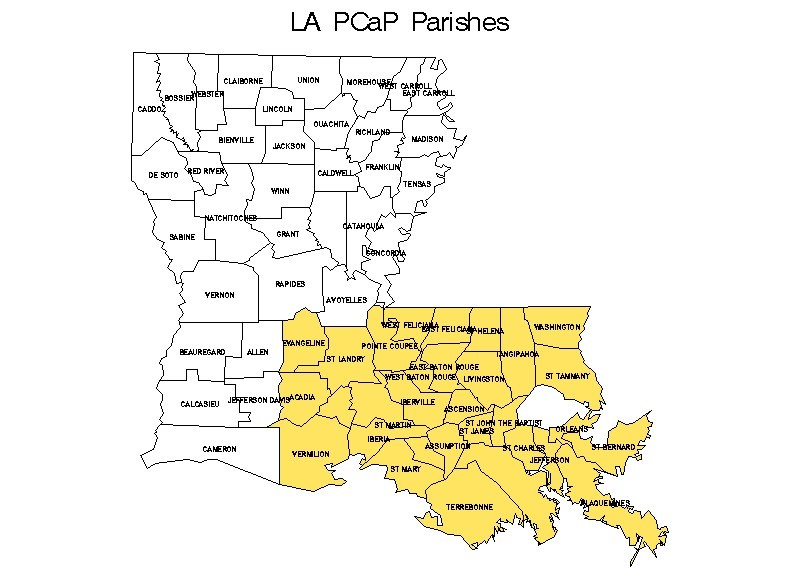
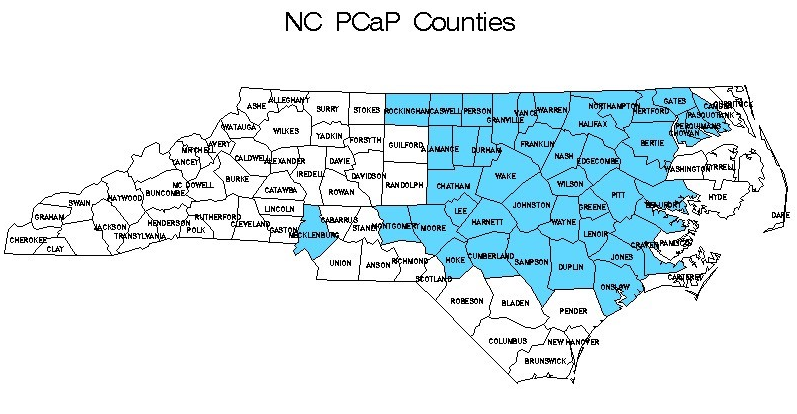
Residents of the North Carolina and Louisiana study areas (see the maps below) with a first diagnosis of histologically confirmed adenocarcinoma of the prostate were eligible to participate if they were diagnosed on or after July 1, 2004 but before the end of recruitment at each site (October 31, 2007 in North Carolina and July 31, 2009 in Louisiana), were 40-79 years old at diagnosis, could complete the study interview in English, did not live in an institution (nursing home), were not cognitively impaired or in a severely debilitated physical state, and were not under the influence of alcohol, severely medicated, or apparently psychotic at the time of the interview. Eligible men also had to self-identify as at least part African American/Black or Caucasian American/White in response to the open-ended interview question “What is your race?”. The cancer registries that provided contact information in each state required that permission be obtained from each patient’s physician before study staff could contact the patient. Enrollment response rates varied by sites and race, as demonstrated in the table here.
Patient Eligibility
Residents of the North Carolina and Louisiana study areas with a first diagnosis of histologically confirmed adenocarcinoma of the prostate were eligible to participate if they were diagnosed on or after July 1, 2004 but before the end of recruitment at each site, were 40-79 years old at diagnosis, could complete the study interview in English, did not live in an institution (nursing home), were not cognitively impaired or in a severely debilitated physical state, and were not under the influence of alcohol, severely medicated, or apparently psychotic at the time of the interview. Eligible men also had to self-identify as at least part African American/Black or Caucasian American/White in response to the open-ended interview question "What is your race?", and a randomized recruitment procedure was used to facilitate comparable recruitment of Caucasian Americans (CA) and African Americans (AA) over time, as described below. The cancer registries that provided contact information in each state required that permission be obtained from each patient's physician before study staff could contact the patient.
North Carolina Case Ascertainment, Enrollment and Participation
The Rapid Case Ascertainment facility of the North Carolina Central Cancer Registry and the Lineberger Comprehensive Cancer Center reported all potentially eligible cases residing in a 42-county study area beginning in October 2003 and ending in August 2007 (for CA) or November 2007 (for AA). Counties included in the study area increased from 27 at the start of enrollment to 42 at the end of the study (see Appendix 2 for study maps and the list of study counties). In total, 4,640 unique cases were reported to the study, including 1,599 AA, 2,860 CA, 171 cases of unknown race and 10 cases that were classified as race other than AA or CA. NC Caucasian Americans were sampled for recruitment with probability of 0.44; consequently, 1,607 CA cases were randomly excluded from recruitment at ascertainment or when their racial status was confirmed to be CA. Cases reported less than 90 days prior to the last day recruitment was attempted (Sept. 17, 2007 for CA, Nov. 29, 2007 for AA) were also excluded from the pool of potentially eligible cases because there was insufficient time to obtain physician permission and complete enrollment (64 AA, 35 CA and 8 men of unknown race).NC cases completed PCaP study visits between October 19, 2004 and December 10, 2007. The average time from diagnosis to the study visit was 164 days (169.5 for AA, 158.8 for CA) with a median time of 134 (range 43 to 831) overall, and 138 (range 48 to 831) in AA, 132 (range 43 to 674) in CA. In total, 1,031 eligible North Carolina men participated in the PCaP study, including 505 AA and 526 CA. The overall response rate, defined as the number of eligible cases enrolled (1,031) divided by the number eligible to participate (2,704), was 38.1%. There were 143 non-participants of unknown race assumed to be eligible (reported as physician refusal (24), deceased (1) no contact (88), lost contact (2), refused (28)). The response rate for AA cases was 35.4% and 46.4% for CAs. The overall participation rate, defined as the number of eligible cases enrolled (1,031) divided by the number enrolled plus the number that refused participation (1,031 + 620) was 62.4%. The participation rate among AA cases was 62.0%, and the participation rate among CA cases was 65.1%.
Louisiana Ascertainment, Enrollment and Participation
Louisiana cases were reported to the study by the Louisiana Tumor Registry. Data for Louisiana are reported separately for cases enrolled prior to Hurricane Katrina and those enrolled after Louisiana field activities resumed in August 2006. In most cases, data will be analyzed separately for pre- and post-Katrina Louisiana cases because of differences in the study populations between the two time periods.
Pre-Katrina
Pre-Katrina Louisiana enrollment included eligible cases diagnosed in 13 parishes surrounding New Orleans, with study visits conducted from September 10, 2004 to August 25, 2005. This period of data collection included study visits with 119 AA and 94 CA participants. During this time, CAs were sampled for recruitment with a probability of 0.42 until March 16, 2005, changing to 0.45 on March 16, 2005. The average time from diagnosis to the study visit was 112.7 days (103.1 for AAs, 125.1 for CAs) with a median time of 94 (range 34 to 304) overall, and 90 (range 34 to 302) in AAs, 106.5 (range 50 to 304) in CAs. The overall response rate defined as the number of eligible cases enrolled (213) divided by the number eligible (479) to participate, was 44.5%. There were 38 non-participants of unknown race assumed to be eligible (reported as physician refusal (7), deceased (2), no contact (6), lost contact (17), refused (6)). The response rate for AA cases was 49.4% and 47.0% for CAs. The overall participation rate, defined as the number of eligible cases enrolled (213) divided by the number enrolled plus the number that refused participation (213 + 82) was 72.2%. The participation rate among AA cases was 70.4%, and the participation rate among CA cases was 78.3%.
Post-Katrina
A second phase of Louisiana enrollment began in an expanded study area (including 8 additional parishes in southern Louisiana, see Appendix 2 for maps and the list of study parishes) on September 5, 2006 and ended on August 31, 2009, after study visits were completed with 506 African American and 508 Caucasian American patients (in addition to the 213 pre-Katrina participants described above). All CA were recruited at the start of post-Katrina field activities because of uncertainties regarding population demographics following Hurricane Katrina, but CA were sampled with a probability of 0.45 between September 5, 2006 and March 29, 2007; 0.55 between March 30, 2007 and January 6, 2008; and 0.44 beginning on January 7, 2008. The average time from diagnosis to the study visit was 140.5 days (143.4 for AA, 137.6 for CA) with a median time of 112 (range 37 to 702) overall, and 109 (range 37 to 702) in AA, 113.5 (range 40 to 526) in CA. The overall response rate defined as the number of eligible cases enrolled (1014) divided by the number eligible (2078) to participate, was 48.8%. There were 163 assumed eligible non-participants of unknown race (reported as physician refusal (11), deceased (1) no contact (50), lost contact (14), refused (87)). The response rate for AA cases was 46.8% and 61.0% for CA. The overall participation rate, defined as the number of eligible cases enrolled (1014) divided by the number enrolled plus the number that refused participation (1014 + 601) was 62.8%. The participation rate among AA cases was 62.5%, and the participation rate among CA cases was 70.7%.