Return to Resources & Protocols page
Guidelines for Use of
Data and Biospecimens from the
North Carolina – Louisiana
Prostate Cancer Project (PCaP)
Last updated September 13, 2012
Funded by the Department of Defense
contract DAMD 17-03-2-0052
Data and Biospecimens from the
North Carolina – Louisiana
Prostate Cancer Project (PCaP)
Last updated September 13, 2012
Funded by the Department of Defense
contract DAMD 17-03-2-0052
TABLE OF CONTENTS
Section
Introduction
Overview of PCaP
Study Questionnaires
Medical Records Retrieval and Abstraction
Biologic Sample Collection, Processing and Storage
Tumor Block Retrieval and Tissue Microrarray (TMA) Construction
Appendix
List of Tables
Table 1. Demographic and background characteristics of PCaP participants
Table 2a. Summary of Biologic Samples Collected from PCaP Subjects
Table 2b. Biospecimen Repository: proportions of subjects with samples available
Table 2c. Biospecimen repository: means (vol/aliquot)
Table 3. Multi-source DNA Specimens: Proportions of subjects with aliquots available
Table 4a. Summary of Diagnostic and Radical Prostatectomy Tissue Collected by Core 1
Table 4b. Diagnostic biopsy and radical prostatectomy specimens processed by Core 3
Table 4c. Total number of subjects on tissue microarrays
Table 4d. Number of subjects and cores on each tissue microarray
Table A1. All reported cases in NC
Table A2. All reported cases in pre-Katrina LA
Table A3. All reported cases in post-Katrina LA
Appendix 3. PCaP essential variables
All tables and summary statistics in this document were generated from a dataset that contains all UNC visits and LSU visits (SAS dataset analysis1a#251.sas7bdat, analysis1a#344.sas7bdat).
Introduction
This document gives an overview of the North Carolina — Louisiana Prostate Cancer Project (PCaP). The study design, study outcomes, tissue collection procedures, and sample availability are described in the sections below, with the list of 13 key demographic and tumor characteristic variables provided in Appendix 3. The PCaP Management Committee is responsible for final decisions regarding the use of the biorepository. Requests to use the PCaP repository can be made by contacting PCaP (pcap@med.unc.edu ).
PCaP does not provide funding to support applicants' projects. Investigators are required to find funding to support their research projects as well as any additional costs for selecting, processing and shipping samples.
[back to top]
Overview of the North Carolina-Louisiana Prostate Cancer Project (PCaP)
The North Carolina-Louisiana Prostate Cancer Project (PCaP) is a multidisciplinary population-based case-only study designed to address racial differences in prostate cancer through a comprehensive evaluation of social, individual and tumor level influences on prostate cancer aggressiveness. PCaP enrolled approximately equal numbers of African Americans and Caucasian Americans with newly-diagnosed prostate cancer from Louisiana and North Carolina. The primary goals of the study are to investigate the factors associated with aggressive prostate cancer in the population as a whole, and compare risk factors for aggressive prostate cancer between the two racial groups. Geographic differences in aggressive prostate cancer within racial groups will also be evaluated to see if differences in race-specific prostate cancer mortality rates between North Carolina and Louisiana (specifically, higher mortality rates for African Americans in North Carolina (NC) versus Louisiana (LA), and higher mortality rates for Caucasian Americans in Louisiana versus North Carolina) can be explained. The PCaP Consortium website is located at https://pcap.bioinf.unc.edu.
[back to top]
Eligibility
Residents of the North Carolina and Louisiana study areas with a first diagnosis of histologically confirmed adenocarcinoma of the prostate were eligible to participate if they were diagnosed on or after July 1, 2004 but before the end of recruitment at each site, were 40-79 years old at diagnosis, could complete the study interview in English, did not live in an institution (nursing home), were not cognitively impaired or in a severely debilitated physical state, and were not under the influence of alcohol, severely medicated, or apparently psychotic at the time of the interview. Eligible men also had to self-identify as at least part African American/Black or Caucasian American/White in response to the open-ended interview question "What is your race?", and a randomized recruitment procedure was used to facilitate comparable recruitment of Caucasian Americans (CA) and African Americans (AA) over time, as described below. The cancer registries that provided contact information in each state required that permission be obtained from each patient's physician before study staff could contact the patient.
North Carolina Case Ascertainment, Enrollment and Participation
The Rapid Case Ascertainment facility of the North Carolina Central Cancer Registry and the Lineberger Comprehensive Cancer Center reported all potentially eligible cases residing in a 42 county study area beginning in October 2003 and ending in August 2007 (for CA) or November 2007 (for AA). Counties included in the study area increased from 27 at the start of enrollment to 42 at the end of the study (see Appendix 2 for study maps and the list of study counties). In total, 4,640 unique cases were reported to the study, including 1,599 AA, 2,860 CA, 171 cases of unknown race and 10 cases that were classified as race other than AA or CA. NC Caucasian Americans were sampled for recruitment with probability of 0.44; consequently, 1,607 CA cases were randomly excluded from recruitment at ascertainment or when their racial status was confirmed to be CA. Cases reported less than 90 days prior to the last day recruitment was attempted (Sept. 17, 2007 for CA, Nov. 29, 2007 for AA) were also excluded from the pool of potentially eligible cases because there was insufficient time to obtain physician permission and complete enrollment (64 AA, 35 CA and 8 men of unknown race).NC cases completed PCaP study visits between October 19, 2004 and December 10, 2007. The average time from diagnosis to the study visit was 164 days (169.5 for AA, 158.8 for CA) with a median time of 134 (range 43 to 831) overall, and 138 (range 48 to 831) in AA, 132 (range 43 to 674) in CA. In total, 1,031 eligible North Carolina men participated in the PCaP study, including 505 AA and 526 CA. The overall response rate, defined as the number of eligible cases enrolled (1,031) divided by the number eligible to participate (2,704), was 38.1%. There were 143 non-participants of unknown race assumed to be eligible (reported as physician refusal (24), deceased (1) no contact (88), lost contact (2), refused (28)). The response rate for AA cases was 35.4% and 46.4% for CAs. The overall participation rate, defined as the number of eligible cases enrolled (1,031) divided by the number enrolled plus the number that refused participation (1,031 + 620) was 62.4%. The participation rate among AA cases was 62.0%, and the participation rate among CA cases was 65.1%. For additional details, see Participation rates summary in Appendix 1.
Louisiana Ascertainment, Enrollment and Participation
Louisiana cases were reported to the study by the Louisiana Tumor Registry. Data for Louisiana are reported separately for cases enrolled prior to Hurricane Katrina and those enrolled after Louisiana field activities resumed in August 2006. In most cases, data will be analyzed separately for pre- and post-Katrina Louisiana cases because of differences in the study populations between the two time periods.
Pre-Katrina
Pre-Katrina Louisiana enrollment included eligible cases diagnosed in 13 parishes surrounding New Orleans, with study visits conducted from September 10, 2004 to August 25, 2005. This period of data collection included study visits with 119 AA and 94 CA participants. During this time, CAs were sampled for recruitment with a probability of 0.42 until March 16, 2005, changing to 0.45 on March 16, 2005. The average time from diagnosis to the study visit was 112.7 days (103.1 for AAs, 125.1 for CAs) with a median time of 94 (range 34 to 304) overall, and 90 (range 34 to 302) in AAs, 106.5 (range 50 to 304) in CAs. The overall response rate defined as the number of eligible cases enrolled (213) divided by the number eligible (479) to participate, was 44.5%. There were 38 non-participants of unknown race assumed to be eligible (reported as physician refusal (7), deceased (2), no contact (6), lost contact (17), refused (6)). The response rate for AA cases was 49.4% and 47.0% for CAs. The overall participation rate, defined as the number of eligible cases enrolled (213) divided by the number enrolled plus the number that refused participation (213 + 82) was 72.2%. The participation rate among AA cases was 70.4%, and the participation rate among CA cases was 78.3%. For additional details, see Participation rates summary in Appendix 1.
Post-Katrina
A second phase of Louisiana enrollment began in an expanded study area (including 8 additional parishes in southern Louisiana, see Appendix 2 for maps and the list of study parishes) on September 5, 2006 and ended on August 31, 2009, after study visits were completed with 506 African American and 508 Caucasian American patients (in addition to the 213 pre-Katrina participants described above). All CA were recruited at the start of post-Katrina field activities because of uncertainties regarding population demographics following Hurricane Katrina, but CA were sampled with a probability of 0.45 between September 5, 2006 and March 29, 2007; 0.55 between March 30, 2007 and January 6, 2008; and 0.44 beginning on January 7, 2008. The average time from diagnosis to the study visit was 140.5 days (143.4 for AA, 137.6 for CA) with a median time of 112 (range 37 to 702) overall, and 109 (range 37 to 702) in AA, 113.5 (range 40 to 526) in CA. The overall response rate defined as the number of eligible cases enrolled (1014) divided by the number eligible (2078) to participate, was 48.8%. There were 163 assumed eligible non-participants of unknown race (reported as physician refusal (11), deceased (1) no contact (50), lost contact (14), refused (87)). The response rate for AA cases was 46.8% and 61.0% for CA. The overall participation rate, defined as the number of eligible cases enrolled (1014) divided by the number enrolled plus the number that refused participation (1014 + 601) was 62.8%. The participation rate among AA cases was 62.5%, and the participation rate among CA cases was 70.7%. For additional details, see Participation rates summary in Appendix 1.
Baseline Data Collection
Participants were visited in their home by a trained Registered Nurse. The study nurse collected biologic samples, made anthropometric measurements and administered the questionnaire. For more information please see manual of procedures for specimen collection, body measurement, or questionnaire.
Table 1. Demographic and background characteristics of PCaP participants
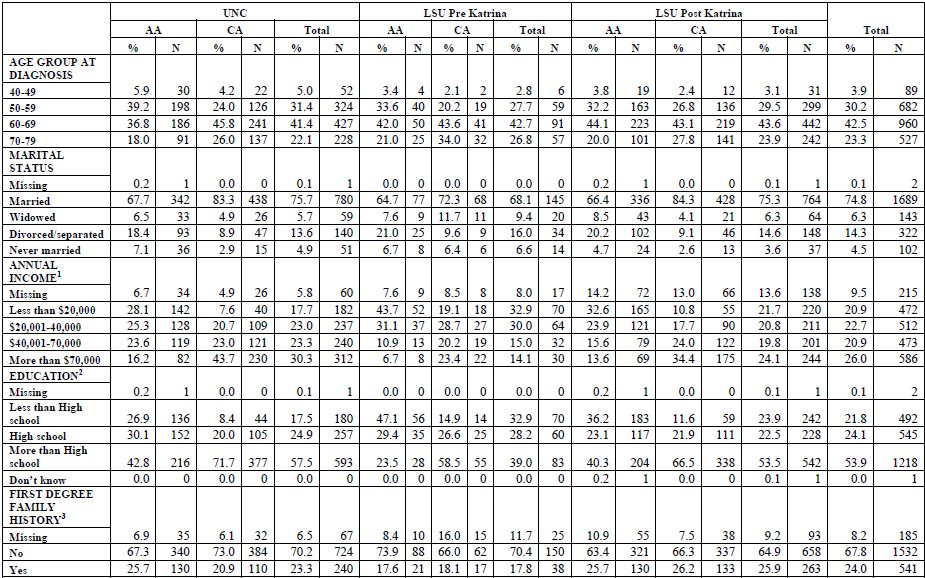
1
Income: Annual household income (before taxes). Missing includes
'refused' and "I don't know" responses.
2 Education: >high school includes participants with vocational/technical training or any college.
3 First degree family history of doctor diagnosed prostate cancer in a father, brother, half-brother, or son.
4 Based on the question "In general, would you say your health is...".
5 Self-reported history of screening PSA blood tests or digital rectal exams (DRE) prior to the diagnosis of prostate cancer, not including any PSA tests or DRE involved in the diagnosis of their prostate cancer. Row PSA includes all patients who reported PSA screening, and did not report DRE or DRE was missing. Row DRE includes patients who reported DRE, and did not report PSA or information on PSA was missing.
6 Self-reported start of treatment prior to visit. Participant's answer to the question "Have you started treatment (such as hormone therapy, radiation therapy, brachytherapy or other) or had surgery?"
7 Aggressiveness of tumor as described in Appendix 3
[back to top]2 Education: >high school includes participants with vocational/technical training or any college.
3 First degree family history of doctor diagnosed prostate cancer in a father, brother, half-brother, or son.
4 Based on the question "In general, would you say your health is...".
5 Self-reported history of screening PSA blood tests or digital rectal exams (DRE) prior to the diagnosis of prostate cancer, not including any PSA tests or DRE involved in the diagnosis of their prostate cancer. Row PSA includes all patients who reported PSA screening, and did not report DRE or DRE was missing. Row DRE includes patients who reported DRE, and did not report PSA or information on PSA was missing.
6 Self-reported start of treatment prior to visit. Participant's answer to the question "Have you started treatment (such as hormone therapy, radiation therapy, brachytherapy or other) or had surgery?"
7 Aggressiveness of tumor as described in Appendix 3
Study Questionnaires
- Background characteristics: self-described race and ethnicity, marital status, religion, education, income, tobacco use, physical activity;
- Occupation: current employment, occupation and industry; longest and second longest occupation and industry; military service; occupations associated with pesticide use;
- Family history: prostate cancer in first- and second-degree relatives;
- Health status: general health (SF-12©) and comorbid conditions;
- Health care: usual sources of care, health insurance, traditional health beliefs, perceived access and quality of care;
- Prostate cancer diagnosis and screening history: PSA tests, digital rectal exams, urinary and sexual symptoms, previous prostate biopsies;
- Medication Survey: All prescription and over-the-counter medications and supplements used in the prior 2 weeks (transcribed by study nurses);
- Non-steroidal anti-inflammatory drugs (NSAIDs): Frequency and duration of use for prescription and over-the-counter NSAIDs taken during the past 5 years at least once a month for one week or longer, with product name show cards to aid recall;
- Vitamins and supplements (including herbal products);
- The diet history questionnaire (DHQ) was developed by the National Cancer Institute and modified by PCaP Project 3 investigators to include Southern foods. Questionnaire responses were linked to the updated DHQ Nutrient Database through the NCI-developed Diet*Calc software to estimate intake of fatty acid and antioxidant micronutrients, including omega-3 and omega-6 polyunsaturated fatty acids, carotenoids, and tocopherols.
- Rapid estimate of adult literacy questionnaire.
- Post-Katrina survey (administered in LA only).
[back to top]
Medical Records Retrieval and Abstraction
Medical records were requested from the physician of consenting participants. Trained staff used a relational database designed specifically for PCaP to abstract information concerning comorbid conditions, family history of prostate cancer, urologic symptoms, indications for diagnostic examinations and biopsies, prostate cancer screening examinations, physical examinations and laboratory assays at or near diagnosis, imaging examinations used in staging, clinical stage and grade, and initial treatment information. The stage was derived for all subjects with medical records according to the algorithm described in Appendix 3. The clinical stage that was assigned by the physician was also abstracted when available. For more details see the Manual of Operations.
[back to top]
Biologic Sample Collection, Processing and Storage
Blood: Approximately 42ml of blood was obtained from consenting participants, including three 8.5ml yellow top (ACD) tubes, one 10 ml red top tube, and one 6.5ml lavender top (EDTA) tube. Red and lavender top tubes were wrapped in foil and were transported on ice prior to initial processing. Serum was removed from the red top tube and aliquoted into ten cryovials. Lavender top samples were processed into plasma (six aliquots) and packed red blood cells (two aliquots). Yellow top tubes were transported at room temperature, and Louisiana samples were shipped overnight to UNC for processing and lymphocyte immortalization. Immortalized lymphocytes were divided into 6 aliquots and cryopreserved in liquid nitrogen. Plasma was removed from yellow-top tubes and DNA was purified from white blood cells. DNA, plasma, serum and packed red blood cells were stored long-term at -80?C.
Buccal rinse: For participants whose DNA was unavailable from blood, a retrospective collection of buccal rinse samples was conducted in NC and LA. Beginning on September 4, 2007, LA participants who, at the time of the study visit, could not complete the blood draw for DNA were given the option to complete a buccal rinse instead.
Urine: A 20ml urine sample was requested from participants. The study nurse immediately aliquoted half of the sample into a 15ml conical centrifuge tube containing 20 mg of crystalline ascorbic acid (as a preservative) and placed the remainder into a second conical tube without preservative. Samples were wrapped in foil and transported on ice prior to long-term storage at -20?C.
Adipose tissue: Subcutaneous fat samples were obtained from the abdominal area of consenting participants who were not allergic to local anesthetics. After the overlying skin was anesthetized with 2% lidocaine solution, a 15-gauge needle was inserted into the subcutaneous fat and suction was applied using a 15ml vacutainer tube. Aspirated tissue was trapped in the needle and Luer lock adapter, which were placed in separate cryovials, transported on ice, and stored at -80?C.
Toenail clippings: Participants were asked to collect toenail clippings from each toe of one foot prior to the study visit. Toenails were stored in a cryovial at ambient temperature.
Anthropometric Measurements: Weight (to the nearest 0.1 kg), height, and waist and hip circumferences (in cm) were measured using standardized instruments.
Diagnostic and Radical Prostatectomy Tissue: See Tumor Block Retrieval and Tissue Microarray (TMA) Construction.
[back to top]
Table 2a. Summary of Biologic Samples Collected from PCaP Subjects
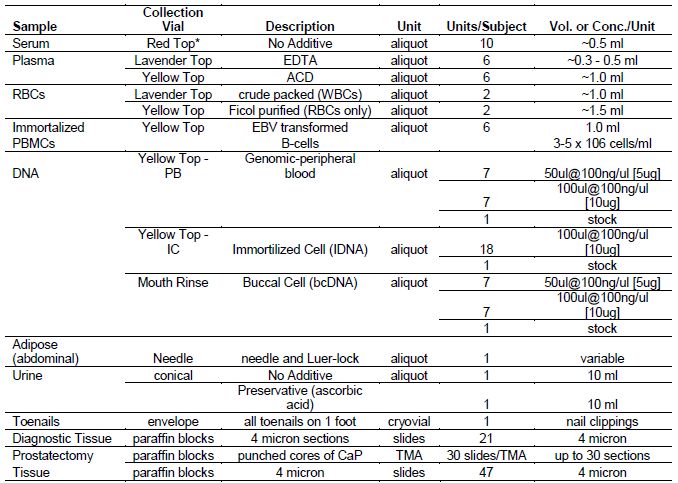
NOTE: In most cases
biological samples were collected after initiation of treatment
* a portion of red top serum tubes were transported from the field at ambient temp at UNC through April 30, 2007; the remaining UNC red top tubes and all of LSU red top tubes were transported on ice
[back to top]* a portion of red top serum tubes were transported from the field at ambient temp at UNC through April 30, 2007; the remaining UNC red top tubes and all of LSU red top tubes were transported on ice
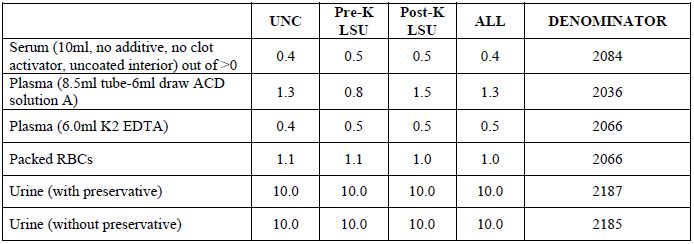
Table 2b. Biospecimen repository: proportions of subjects with samples2

1
There were 835 UNC subjects (395 AA, 440 CA) interviewed before May 1,
2007. Of these subjects, 772 (360 AA, 412 CA) contributed serum
samples, which were transported at room temperature rather than on ice.
2 For more detail on specimen preparation, see manual of operations.
[back to top]2 For more detail on specimen preparation, see manual of operations.
Table 2c. Biospecimen repository: means (vol/aliquot)
Type I error
Power
[back to top]Power
Table 3. Multi-source DNA Specimens: proportions of subjects with aliquots available
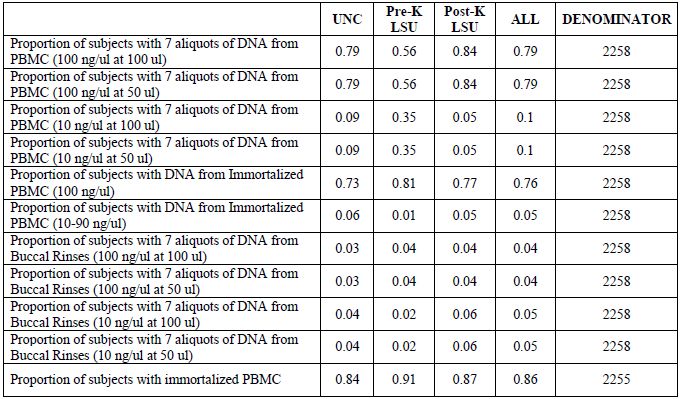
[back to top]
Tumor Block Retrieval and Tissue Microrarray (TMA) Construction
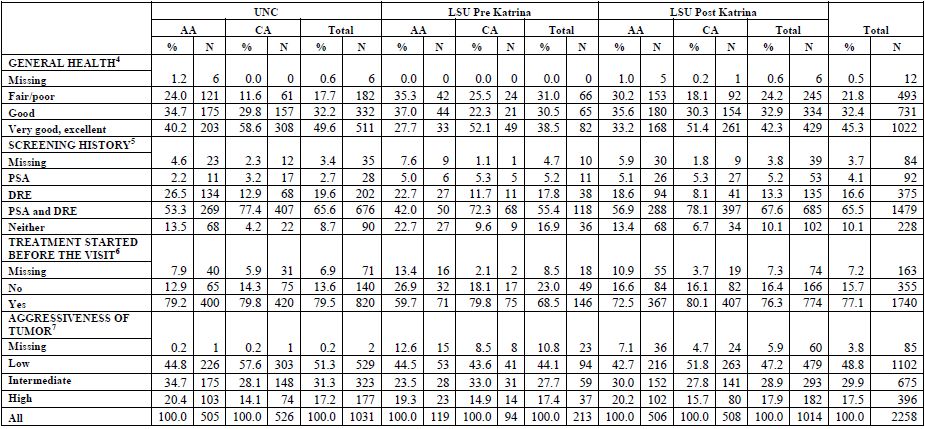
Tumor block retrieval is summarized in Table 4a. In nearly all state-race groups, 100% of subjects who completed a study visit also consented to release diagnostic tissue. The exceptions are 99.8% of post-Katrina LA AA (505 of 508) and 99.4% of NC AA (502 of 505). Paraffin blocks containing diagnostic prostate tissue biopsies (core biopsies or TURP samples used to diagnose cancer) were requested from hospitals or reference pathology laboratories. A few facilities provided unstained slides instead of tissue blocks. Hospitals/labs declined to release diagnostic tissue for 96 or 9.3% of NC cases (56 or 11.2% AA, 40 or 7.6% CA), 44 or 20.7% of pre-Katrina cases (32 or 26.9% AA, 12 or 12.8% CA) and 96 or 9.5% post-Katrina LA cases (81 or 16.0% AA, 15 or 3.0% CA). Reasons for decline included overall policy (i.e., no blocks are ever released from that facility) or case-specific reasons (e.g., a particular patient's blocks did not contain enough tissue).
Diagnostic tissue (blocks or slides) was received for 879 NC subjects or 85.3% of NC subjects who completed a visit (412 or 81.6% AA; 467 or 88.8% CA); 113 or 53.1% of pre-Katrina subjects (53 or 44.5% AA, 60 or 63.8% CA); and 775 or 76.4% of post-Katrina subjects (363 or 71.7% AA, 412 or 81.1% CA).
For each subject, six sections were cut from each representative positive diagnostic block and one from each negative (without adenocarcinoma) block. Of the six sections from each positive diagnostic block; one was stained for eosin and hematoxylin and reviewed by the study pathologist in order to identify areas of cancer. One additional positive section/slide was immuno-stained for Ki-67 and caspase-3 and analyzed using automated image analysis. The remaining sections were archived. See Table 4b for additional detail.
In NC, 638 subjects (289 AA, 349 CA) indicated at the time of the study visit that they chose radical prostatectomy (RP) for their method of prostate cancer treatment. In pre-Katrina LA, 99 subjects (58 AA, 41 CA) chose RP, while in post-Katrina LA 509 subjects (230 AA, 279 CA) chose RP. This equates to 61.9% of NC subjects who completed a study visit (57.2% AA, 66.3% CA), 46.5% of pre-Katrina subjects (48.7% AA, 43.6% CA) and 50.2% of post-Katrina subjects (45.5% AA, 54.9% CA). Of those subjects who chose RP, consent to release that tissue to PCaP was provided by 593 or 92.9% of NC subjects (260 or 90.0% AA, 333 or 95.4% CA), 50 or 50.5% of pre-Katrina subjects (23 or 39.7% AA, 27 or 65.9% CA), and 506 or 99.4% of post-Katrina subjects (228 or 99.1% AA, 278 or 99.6% CA). Some NC subjects and all pre-Katrina LA subjects were contacted after the study visit to ask for release of RP tissue, with many pre-Katrina LA subjects lost to contact after Hurricane Katrina; thus, their rates are lower than those for post-Katrina LA, all of whom were asked for release of RP tissue at the time of their study visit.
Paraffin-embedded prostatectomy specimens were requested for consenting participants who had undergone or scheduled an RP at the time of the study visit. A few facilities provided unstained slides instead of tissue blocks. Hospitals/labs declined to release RP tissue for 68 or 11.5% NC subjects (23 or 8.8% AA, 45 or 13.5 % CA), 7 or 14.0% pre-Katrina (3 or 13.0% AA, 4 or 14.8% CA) and 38 or 7.5% post-Katrina LA subjects (13 or 5.7% AA, 25 or 9.0% CA). Reasons for decline included overall policy (i.e., no blocks were ever released from that facility) or case-specific reasons (e.g., a particular patient's blocks did not contain enough tissue).
RP tissue was received for 442 NC subjects or 42.9% of NC subjects who completed a visit (196 or 38.8% AA, 246 or 46.8% CA); 19 or 8.9% of pre-Katrina subjects (9 or 7.6% AA, 10 or 10.6% CA); and 331 or 32.6% of post-Katrina subjects (169 or 33.4% AA, 162 or 31.9% CA). When considering only those subjects who indicated at the study visit that they chose CaP treatment via RP, rates of RP tissue receipt are 69.3% for NC (67.8% AA, 70.5% CA), 19.2% for pre-Katrina LA (15.5% AA, 24.4% CA) and 65.0% for post-Katrina LA (73.5% AA, 58.1% CA).
Similar to the process for sectioning diagnostic specimens, 6 slides were cut from each positive RP block and 1 from each negative (without adenocarcinoma) RP block. One newly-cut section from the surgical specimen was reviewed by the study pathologist to select areas of cancer for construction of tissue microarrays (TMAs), and additional sections were archived (see Table 4b for additional detail). TMAs contain tissue from an average of 62 subjects. TMAs were constructed with 0.6 mm core punches of neoplastic tissue and one core of paired non-neoplastic tissue from the same subject using the following algorithm:
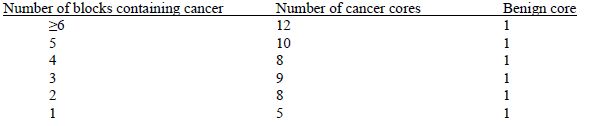
The following information was recorded for each core inserted:
- Research subject study ID number;
- Research Block number;
- Punch identifier;
- Benign or cancer (Gleason primary and secondary grades) coded centrally by a study reference pathologist;
- Percent prostatectomy specimen involved by tumor coded centrally by a study reference pathologist;
- TMA block number (for radical prostatectomy TMAs);
- TMA location (x, y-coordinates) (for radical prostatectomy TMAs).
[back to top]
Table 4a. Summary of Diagnostic and Radical Prostatectomy Tissue Collected by Core 1
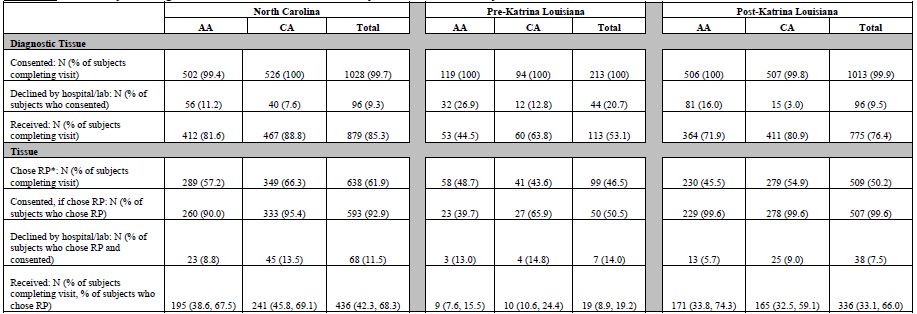
* According to self-report
at the study visit that RP had been performed or was planned
[back to top]Table 4b. Diagnostic biopsy (DX) and radical prostatectomy (RP) specimens processed by Core 32
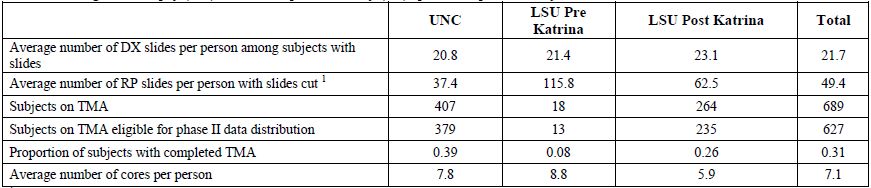
1
Every block received by LSU is cut; at UNC only blocks with cancer are
cut. This results in increased number of blocks cut at LSU.
2 For more detail on tissue/microarray preparation, see manual of operations.
[back to top]2 For more detail on tissue/microarray preparation, see manual of operations.
Table 4c. Total number of subjects on tissue microarrays
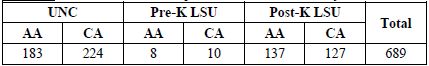
[back to top]
Table 4d. Number of subjects and cores on each tissue microarray
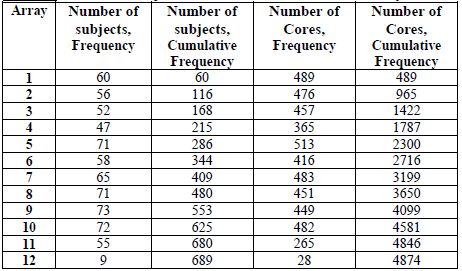
[back to top]
Appendix
Appendix 1. Participation rates summary for NC, pre-Katrina LA and post-Katrina LA
North Carolina PCaP Study Participation
Ineligible
NC Caucasian Americans were sampled for recruitment with probability of 0.44; consequently, 1,607 CA cases were randomly excluded from recruitment at ascertainment or when their racial status was confirmed to be CA. Cases reported less than 90 days prior to the last day recruitment was attempted (Sept. 17, 2007 for CA, Nov. 29, 2007 for AA) were also excluded from the pool of potentially eligible cases because there was insufficient time to obtain physician permission and complete enrollment (64 AA, 35 CA and 8 men of unknown race). Cases reported to the study that were later determined to be ineligible for reasons other than random exclusion included 107 AA, 85 CA, and 20 men of unknown race. Ten men were ineligible because they were determined to be neither AA nor CA.
No contact
Cases were classified as "presumed eligible, no contact" if they were ascertained at least 90 days prior to the last day that recruitment was attempted (389 AA, 223 CA, 88 of unknown race).
Deceased
Cases that died before they could be contacted for enrollment included 6 AA, 3 CA and 1 case of unknown race.
Lost contact
Study visits were never completed with 136 cases that were contacted at least once by telephone, including 94 AA, 40 CA and 2 cases of unknown race.
Physician Permission to Contact Case Denied
The North Carolina Central Cancer Registry required that PCaP obtain permission from diagnosing physicians before attempting recruitment. Therefore, we asked physicians to contact the study within three weeks if they did not want their patient contacted for any reason, including ineligibility due to mental illness or impairment, nursing home residence, or severe physical debilitation. A modified protocol was required for Duke University Medical Center (DUMC) patients, whereby a DUMC employee obtained active consent from patients before DUMC physicians gave PCaP permission to contact them. Approximately 93% of physicians with cases reported and sampled for recruitment provided active or passive consent for PCaP to contact all of their patients. Other physicians refused consent to contact at least one patient, but only three refused consent to contact any of their patients; consequently, it is likely that permission to contact many patients was denied because the patient was ineligible. Nonetheless, we assumed that all participants for whom physician permission for contact was refused would have been eligible unless they were known to be ineligible based on other information, leaving 124 AA, 59 CA, and 24 cases of unknown race classified as "assumed eligible, physician refusal".
Case Refusals
Men who declined to participate in the study when contacted for enrollment included 310 AA, 282 CA, and 28 of unknown race eligible for randomized recruitment.
NC Final Participation Rates
In total, 1,031 eligible North Carolina men participated in the PCaP study, including 505 AA and 526 CA. The overall response rate, defined as the number of eligible cases enrolled (N = 1,031) divided by the number of eligible to participate (1,031 participants + 10 deceased + 700 no contact + 136 lost contact + 620 case refusals + 207 physician refusals) was 38.1%. The response rate for AAs was 35.4% (505 participants / (505 + 6 deceased + 389 no contact + 94 lost contact + 310 case refusals + 124 physician refusals)). The response rate for CAs was 46.4% (526 participants / (526 + 3 deceased + 223 no contact + 40 lost contact + 282 case refusals + 59 physician refusals)).
The overall participation rate, defined as the number of eligible cases enrolled (1,031) divided by the number enrolled plus the number that refused participation (1,031+ 620) was 62.4%. The participation rate for AAs was 62.0% (505 / (505 + 310)). The participation rate for CAs was 65.1% (526 / (526 + 282)). For additional details, see Table A1 below.
Table A1. All reported cases in NC
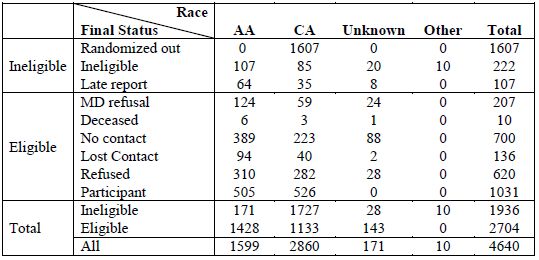
[back to top]
Louisiana PCaP Study Participation
Ineligible
Pre-Katrina CAs were sampled for recruitment with a probability of 0.42 until March 16, 2005, changing to 0.45 on March 16, 2005; consequently, 133 CA cases were randomly excluded from recruitment at ascertainment or when their racial status was confirmed to be CA. All CA were recruited at the start of post-Katrina field activities because of uncertainties regarding population demographics following Hurricane Katrina, but CA were sampled with a probability of 0.45 between September 5, 2006 and March 29, 2007; 0.55 between March 30, 2007 and January 6, 2008; and 0.44 beginning on January 7, 2008. This resulted in the random exclusion of 491 post-Katrina CAs. Post-Katrina cases reported less than 90 days prior to the last day recruitment was attempted (November 11, 2008 for CA; August 27, 2009 for AA) were also excluded from the pool of potentially eligible cases because there was insufficient time to obtain physician permission and complete enrollment (58 AAs, 24 CA and 15 men of unknown race). Cases reported to the study that were later determined to be ineligible for reasons other than random exclusion included: (Pre-Katrina) 14 AA, 17 CA, and 16 men of unknown race; (Post-Katrina) 81 AA, 89 CA and 38 men of unknown race. Five men were ineligible because they were determined to be neither AA nor CA.
No contact
Cases were classified as "presumed eligible, no contact" if they were ascertained at least 90 days prior to the last day that recruitment was attempted (Pre-Katrina: 2 AA, 26 CA, 6 of unknown race; Post-Katrina: 166 AA, 50 CA, 50 of unknown race).
Deceased
Cases that died before they could be contacted for enrollment included 1 AA and 2 unknown race Pre-Katrina cases and 7 AAs, 5 CAs and 1 unknown race Post-Katrina case.
Lost contact
Study visits were never completed with 130 Pre-Katrina cases that were contacted at least once by telephone (65 AA, 48 CA and 17 cases of unknown race); and 114 Post-Katrina cases (72 AAs, 28 CAs and 14 cases of unknown race).
Physician Permission to Contact Case Denied
It was assumed that all participants for whom physician permission for contact was refused would have been eligible unless they were known to be ineligible based on other information, leaving 4 AA, 6 CA, and 17 unknown race Pre-Katrina cases and 28 AAs, 31 CAs, and 11 unknown race Post-Katrina cases classified as "assumed eligible, physician refusal".
Case Refusals
Men who declined to participate in the study when contacted for enrollment included: (Pre-Katrina) 50 AAs, 26 CAs, and 6 of unknown race and (Post-Katrina) 303 AAs, 211 CAs, and 87 of unknown race eligible for randomized recruitment
Pre-Katrina Louisiana Final Participation Rates
In total, 213 eligible pre-Katrina men participated in PCaP, including 119 AA and 94 CA. The overall response rate, defined as the number of eligible cases enrolled (N = 213) divided by the number of eligible to participate (213 participants + 3 deceased + 34 no contact + 130 lost contact + 82 case refusals + 17 physician refusals) was 44.5%. The response rate for AAs was 49.4% (119 participants / (119 + 1 deceased + 2 no contact + 65 lost contact + 50 case refusals + 4 physician refusals)). The response rate for CAs was 47.0% (94 participants / (94 + 0 deceased + 26 no contact + 48 lost contact + 26 case refusals + 6 physician refusals)).
The overall participation rate, defined as the number of eligible cases enrolled (213) divided by the number enrolled plus the number that refused participation (213 + 82) was 72.2%. The participation rate for AAs was 70.4% (119 / (119 + 50)). The participation rate among CAs was 78.3% (94 / (94 + 26)).
For additional details, see Table A2.
Table A2. All reported cases in pre-Katrina LA

[back to top]
Post-Katrina Louisiana Final Participation Rates
In total, 1014 eligible post-Katrina men participated in PCaP, including 506 AA and 508 CA. The overall response rate, defined as the number of eligible cases enrolled (N = 1014) divided by the number of eligible to participate (1014 participants + 13 deceased + 266 no contact + 114 lost to contact + 601 case refusals + 70 physician refusals) was 48.8%. The response rate for AAs was 46.8% (506 participants / (506 + 7 deceased + 166 no contact + 72 lost contact + 303 case refusals + 28 physician refusals)). The response rate for CAs was 61.0% (508 participants / (508 + 5 deceased + 50 no contact + 28 lost contact + 211 case refusals + 31 physician refusals)).
The overall participation rate, defined as the number of eligible cases enrolled (1014) divided by the number enrolled plus the number that refused participation (1014 + 601) was 62.8%. The participation rate for AAs was 62.5% (506 / (506 + 303)). The participation rate among CAs was 70.7% (508 / (508 + 211)). For additional details, see Table A3.
Table A3. All reported cases in post-Katrina LA
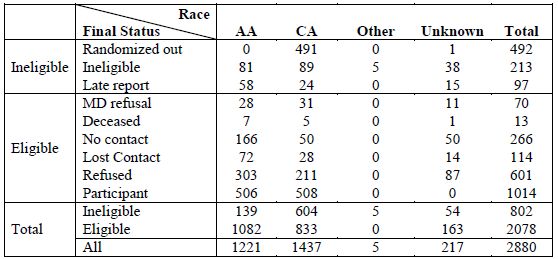
[back to top]
Appendix 2. Participating NC counties and LA parishes
Original PCaP NC counties
Alamance, Caswell, Chatham, Cumberland, Duplin, Durham, Edgecomb, Franklin, Granville, Halifax, Harnett, Hoke, Johnston, Lee, Lenoir, Mecklenburg, Nash, Orange, Person, Pitt, Rockingham, Sampson, Vance, Wake, Warren, Wayne, Wilson.
Expansion PCaP NC counties
Beaufort, Bertie, Chowan, Craven, Gates, Greene, Hertford, Jones, Martin, Montgomery, Moore, Northampton, Onslow, Pasquotank, Perquimans.

Original PCaP LA parishes
Assumption, Jefferson, Lafourche, Orleans, Plaquemines, St. Bernard, St. Charles, St. James, St. John the Baptist, St. Tammany, Tangipahoa, Terrebonne, Washington.
Expansion PCaP LA parishes
Acadia, Ascension, East Baton Rouge, East Feliciana, Evangeline, Iberia, Iberville, Lafayette, Livingston, Pointe Coupee, St. Helena, St. Landry, St. Martin, St. Mary, Vermillion, West Baton Rouge, West Feliciana.

[back to top]
Appendix 3. PCaP essential variables
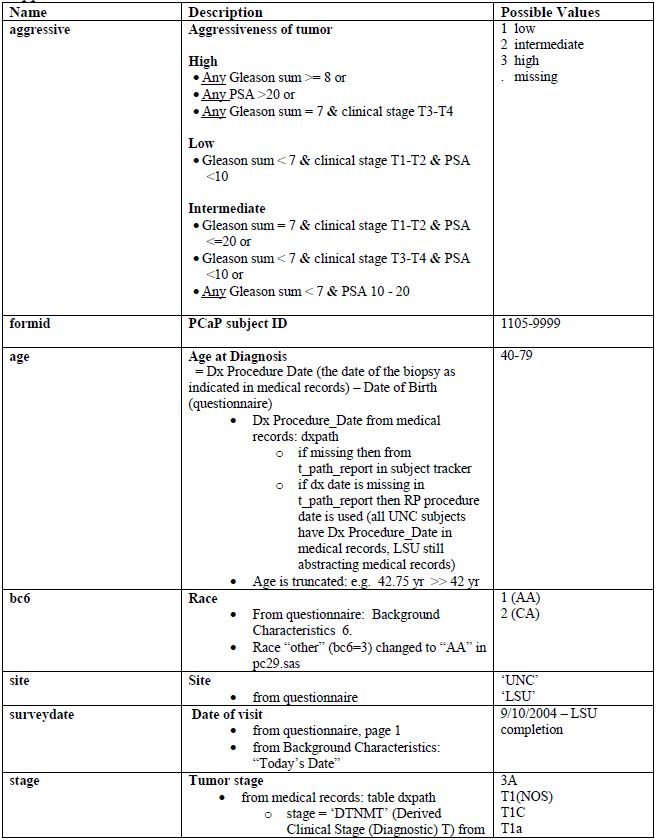
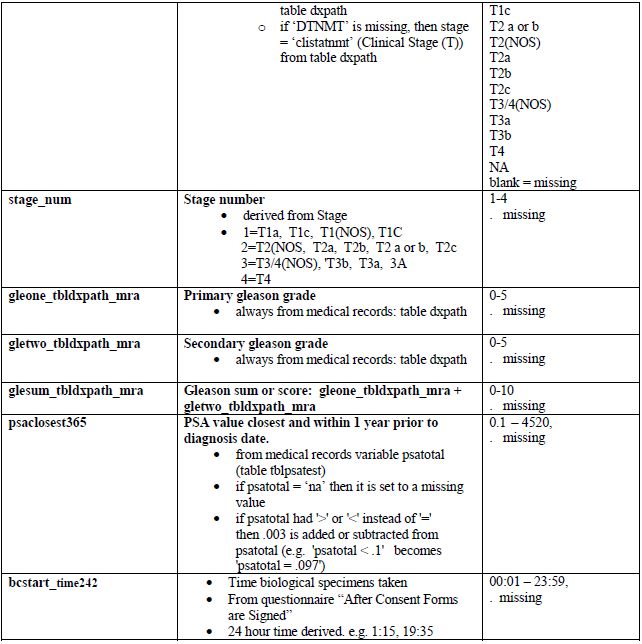
Comments
- Gleason grades and sums (scores) are recorded from the subject's medical record. If no Gleason sum is reported in the diagnostic biopsy table, Gleone, Gletwo and Glesum are missing. Note that for most subjects who had a prostatectomy, a second set of Gleason grades and sum is also available.
- The Gleason grades and sum are reported as indicated in a medical record. These are: the 1° Gleason grade or pattern (assigned to the most predominant histologic pattern in the sample and listed first), the 2° Gleason grade or pattern (assigned to the second most predominant histologic pattern in the sample and listed second) and the Gleason sum or score (the sum of the 1° and 2° Gleason grades, which may sometimes be the only value listed). The primary grade is the first pattern of the sum. If there are more than one positive sites, the pattern with highest Gleason sum is reported. If the sums are the same, the one with the higher primary grade is reported. For example, if someone has three positive sites: 3+3=6, 3+4=7, and 4+3=7, the Gleason 4+3=7 is reported and the 4 is the primary. See Medical Records Abstraction Manual of Procedures for more information.
- The PSA range of 365 days prior to diagnosis was set by the PCaP investigators.




