PCaP Snapshot
Choose here for a printable document formatted for printing.
CLICK ON ANY OF THE TABLES BELOW TO SEE A LARGE FORMAT VERSION.
From the Guidelines for Use of Data and Biospecimens
North Carolina – Louisiana Prostate Cancer Project (PCaP)
Funded by the Department of Defense contract DAMD 17-03-2-0052
See Data
Use Guide
for more detail.
Baseline Data Collection
Participants were visited in their home by a trained Registered Nurse. The study nurse collected biologic samples, made anthropometric measurements and administered the questionnaire. For more information please see manual of procedures for specimen collection, body measurement, or questionnaire.
Overview of the North Carolina-Louisiana Prostate Cancer Project (PCaP)
The North Carolina-Louisiana Prostate Cancer Project (PCaP) is a multidisciplinary population-based case-only study designed to address racial differences in prostate cancer through a comprehensive evaluation of social, individual and tumor level influences on prostate cancer aggressiveness. PCaP enrolled approximately equal numbers of African Americans and Caucasian Americans with newly-diagnosed prostate cancer from Louisiana and North Carolina. The primary goals of the study are to investigate the factors associated with aggressive prostate cancer in the population as a whole, and compare risk factors for aggressive prostate cancer between the two racial groups. Geographic differences in aggressive prostate cancer within racial groups will also be evaluated to see if differences in race-specific prostate cancer mortality rates between North Carolina and Louisiana (specifically, higher mortality rates for African Americans in North Carolina (NC) versus Louisiana (LA), and higher mortality rates for Caucasian Americans in Louisiana versus North Carolina) can be explained.
For more detailed information please see the Data Use Guide.
PCaP Snapshot Sections
Table 1. Demographic and background characteristics of PCaP participants
Medical Records Retrieval and Abstraction
Biologic Sample Collection, Processing and Storage
Table 2a. Summary of Biologic Samples Collected from PCaP Subjects
Table 2b. Biospecimen repository: proportions of subjects with samples
Table 2c. Biospecimen repository: means
Appendix 3. PCaP essential variables
Table 1. Demographic and background characteristics of PCaP participants
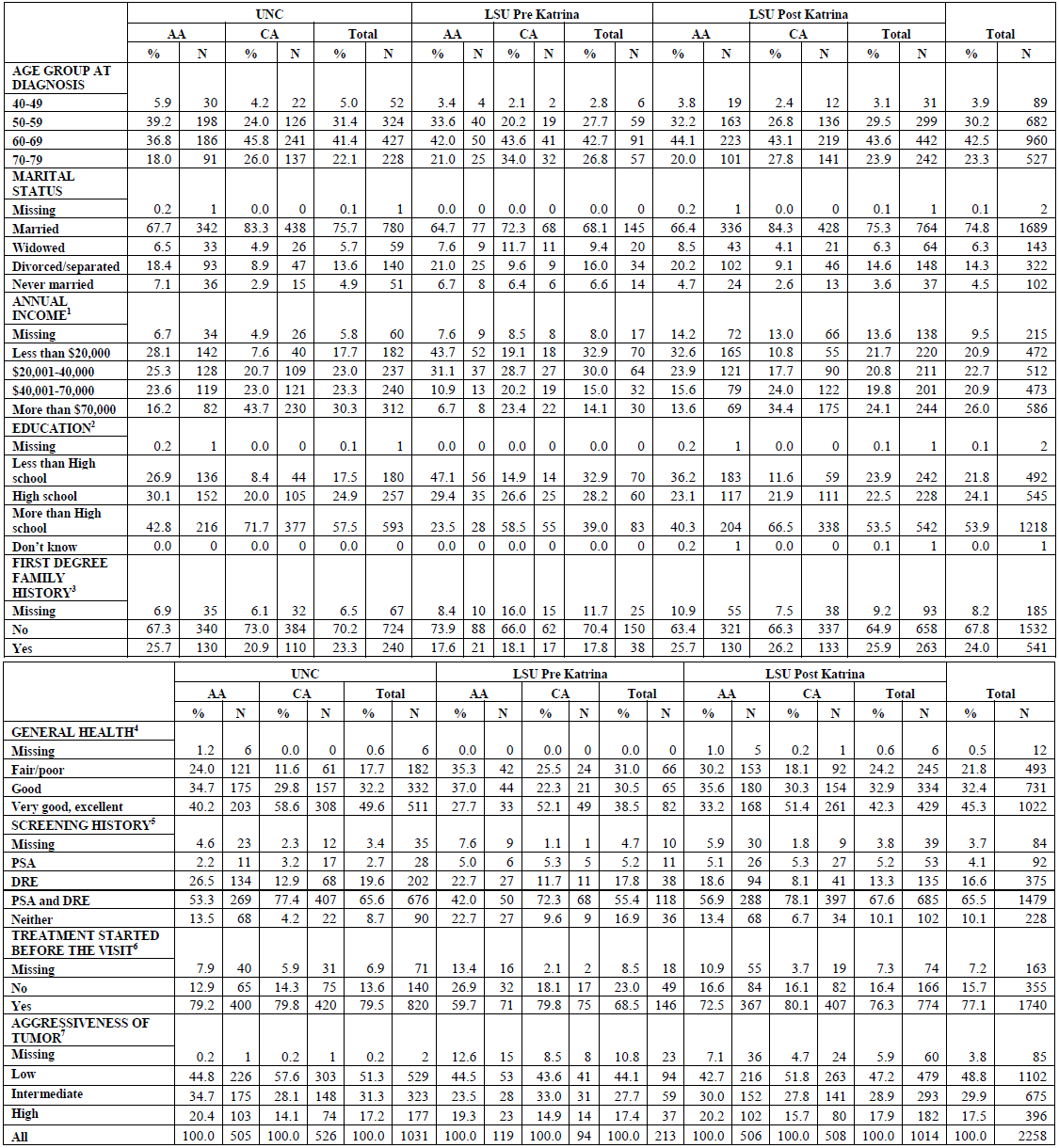
[back to top]
Medical Records Retrieval and Abstraction
Medical records were requested from the physician of consenting participants. Trained staff used a relational database designed specifically for PCaP to abstract information concerning comorbid conditions, family history of prostate cancer, urologic symptoms, indications for diagnostic examinations and biopsies, prostate cancer screening examinations, physical examinations and laboratory assays at or near diagnosis, imaging examinations used in staging, clinical stage and grade, and initial treatment information. The stage was derived for all subjects with medical records according to the algorithm described in Appendix 3. The clinical stage that was assigned by the physician was also abstracted when available. For more details see the Manual of Operations.
[back to top]
Biologic Sample Collection, Processing and Storage
Blood: Approximately 42ml of blood was obtained from consenting participants, including three 8.5ml yellow top (ACD) tubes, one 10 ml red top tube, and one 6.5ml lavender top (EDTA) tube. Red and lavender top tubes were wrapped in foil and were transported on ice prior to initial processing. Serum was removed from the red top tube and aliquoted into ten cryovials. Lavender top samples were processed into plasma (six aliquots) and packed red blood cells (two aliquots). Yellow top tubes were transported at room temperature, and Louisiana samples were shipped overnight to UNC for processing and lymphocyte immortalization. Immortalized lymphocytes were divided into 6 aliquots and cryopreserved in liquid nitrogen. Plasma was removed from yellow-top tubes and DNA was purified from white blood cells. DNA, plasma, serum and packed red blood cells were stored long-term at -80°C.
Buccal rinse: For participants whose DNA was unavailable from blood, a retrospective collection of buccal rinse samples was conducted in NC and LA. Beginning on September 4, 2007, LA participants who, at the time of the study visit, could not complete the blood draw for DNA were given the option to complete a buccal rinse instead.
Urine: A 20ml urine sample was requested from participants. The study nurse immediately aliquoted half of the sample into a 15ml conical centrifuge tube containing 20 mg of crystalline ascorbic acid (as a preservative) and placed the remainder into a second conical tube without preservative. Samples were wrapped in foil and transported on ice prior to long-term storage at -20°C.
Adipose tissue: Subcutaneous fat samples were obtained from the abdominal area of consenting participants who were not allergic to local anesthetics. After the overlying skin was anesthetized with 2% lidocaine solution, a 15-gauge needle was inserted into the subcutaneous fat and suction was applied using a 15ml vacutainer tube. Aspirated tissue was trapped in the needle and Luer lock adapter, which were placed in separate cryovials, transported on ice, and stored at -80°C.
Toenail clippings: Participants were asked to collect toenail clippings from each toe of one foot prior to the study visit. Toenails were stored in a cryovial at ambient temperature.
Anthropometric Measurements: Weight (to the nearest 0.1 kg), height, and waist and hip circumferences (in cm) were measured using standardized instruments.
Diagnostic and Radical Prostatectomy Tissue: See Tumor Block Retrieval and Tissue Microarray (TMA) Construction.
[back to top]
Table 2a. Summary of Biologic Samples Collected from PCaP Subjects
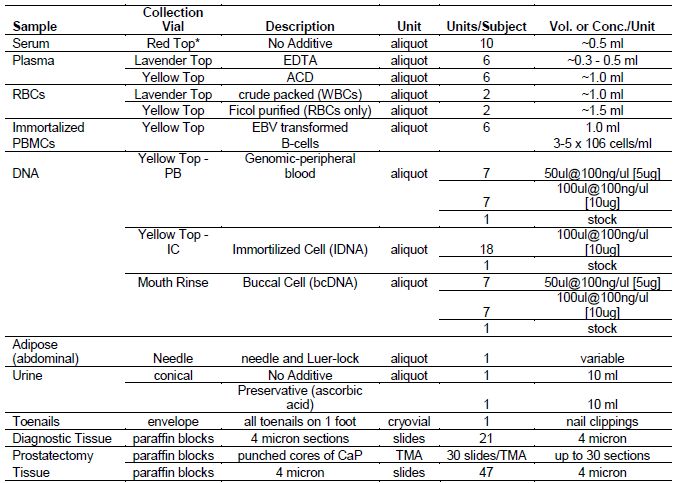
NOTE: In most cases biological samples were collected after initiation of treatment
* a portion of red top serum tubes were transported from the field at ambient temp at UNC through April 30, 2007; the remaining UNC red top tubes and all of LSU red top tubes were transported on ice
[back to top]
Table 2b. Biospecimen repository: proportions of subjects with samples2
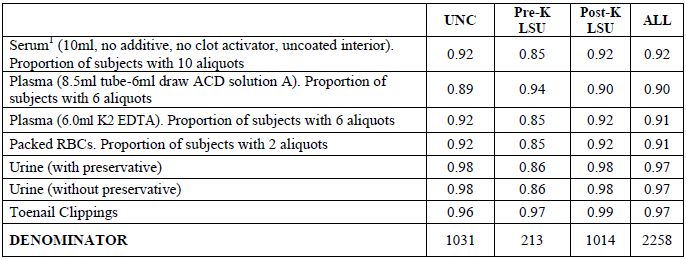
1There were 835 UNC subjects (395 AA, 440 CA) interviewed before May 1, 2007. Of these subjects, 772 (360 AA, 41 CA) contributed serum samples, which were transported at room temperature rather than on ice.
2For more detail on specimen preparation, see manual of operations.
[back to top]
Table 2c. Biospecimen repository: means (vol/aliquot)
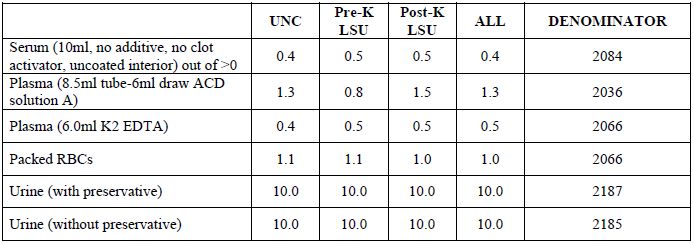
[back to top]
Appendix 3. PCaP essential variables
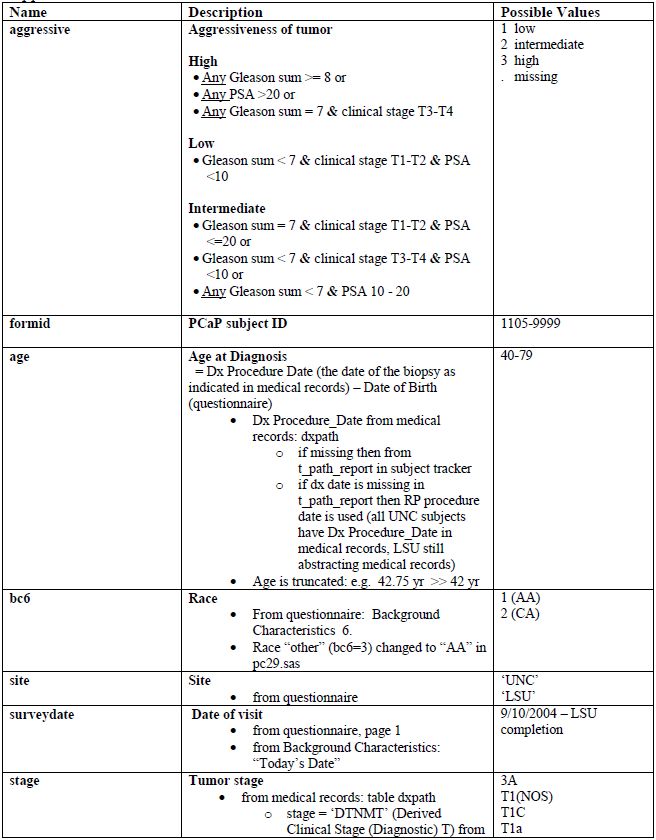
[back to top]




